Simulation Educators provides professional customized training and consulting for modeling and simulation concepts and tools. This blog discusses instructional concepts and issues in modeling and simulation, discrete event simulation, and combat modeling.
Monday, September 12, 2011
Weird Scientists
Sunday, August 21, 2011
Weird Scientist Number 3: Erwin Schrödinger
“God knows I am no friend of probability theory, I have hated it from the first moment when our dear friend Max Born gave it birth. For it could be seen how easy and simple it made everything, in principle, everything ironed and the true problems concealed. Everybody must jump on the bandwagon [Ausweg]. And actually not a year passed before it became an official credo, and it still is[i].” [1]
 Maybe Erwin Rudolf Josef Alexander Schrödinger did not care much for probability theory, but he could not avoid it when trying to formulate his wave theory—and the Schrödinger equation—for subatomic particles. Schrödinger (12 August 1887 – 4 January 1961) was a physicist and theoretical biologist who was one of the fathers of quantum mechanics, and is famed for a number of important contributions to physics, especially the Schrödinger equation, for which he received the Nobel Prize in Physics in 1933. In 1935, after extensive correspondence with friend Albert Einstein, he proposed the Schrödinger’s cat thought experiment[ii].
Maybe Erwin Rudolf Josef Alexander Schrödinger did not care much for probability theory, but he could not avoid it when trying to formulate his wave theory—and the Schrödinger equation—for subatomic particles. Schrödinger (12 August 1887 – 4 January 1961) was a physicist and theoretical biologist who was one of the fathers of quantum mechanics, and is famed for a number of important contributions to physics, especially the Schrödinger equation, for which he received the Nobel Prize in Physics in 1933. In 1935, after extensive correspondence with friend Albert Einstein, he proposed the Schrödinger’s cat thought experiment[ii]. Middle years
Later years
“I am very astonished that the scientific picture of the real world around me is deficient. It gives a lot of factual information, puts all our experience in a magnificently consistent order, but it is ghastly silent about all and sundry that is really near to our heart, that really matters to us. It cannot tell us a word about red and blue, bitter and sweet, physical pain and physical delight; it knows nothing of beautiful and ugly, good or bad, God and eternity. Science sometimes pretends to answer questions in these domains, but the answers are very often so silly that we are not inclined to take them seriously.”
The request for March stemmed from Schrödinger’s unconventional relationships with women: although his relations with his wife Anny were good, he had had many lovers with his wife’s full knowledge (and in fact, Anny had her own lover, Hermann Weyl). Schrödinger asked for March to be his assistant because, at that time, he was in love with March’s wife Hilde.
· “Grundlinien einer Theorie der Farbenmetrik im Tagessehen,” Annalen der Physik, (4), 63, (1920), 397-426; 427-456; 481-520 (Outline of a theory of color measurement for daylight vision)
· “Farbenmetrik,” Zeitschrift für Physik, 1, (1920), 459-466 (Color measurement).
The second of these is available in English as “Outline of a Theory of Color Measurement for Daylight Vision” in Sources of Color Science, Ed. David L. MacAdam, The MIT Press (1970), 134-182.
[2] Nobelprize.org., Erwin Schrödinger - Biography. The Nobel Prize in Physics 1933. [Online] The Nobel Foundation, 1933. [Cited: July 20, 2011.] http://nobelprize.org/nobel_prizes/physics/laureates/1933/schrodinger-bio.html.
[3] Moore, Walter J., A Life of Erwin Schrödinger. Abridged edition. s.l. : Cambridge University Press, 1994. ISBN-13: 978-0521469340.
[4] O'Connor, J. J. and Robertson, E. F., Erwin Rudolf Josef Alexander Schrödinger. School of Mathematics and Statistics, University of St Andrews, Scotland . 2003. http://www-history.mcs.st-andrews.ac.uk/Biographies/Schrodinger.html. Retrieved 20 July 2011..
[5] Schrödinger, Erwin., What is Life?: with "Mind and Matter" and "Autobiographical Sketches". s.l. : Cambridge University Press, 1992. ISBN-13: 978-0521427081.
[6] Meÿenn, Karl., "Schrödinger, Erwin Rudolf Josef Alexander." Neue Deutsche Biographie. online version, 2007, Vol. 23, pp. 578-580. http://www.deutsche-biographie.de/pnd118823574.html.
[7] Muller, H. J., The American Naturalist: V.56 1922. s.l. : University of Michigan Library, 2001 [1929]. ASIN: B002IKLF18 .
[8] Schrödinger, Erwin., 'Nature and the Greeks' and 'Science and Humanism'. s.l. : Cambridge University Press, 1996. ISBN-13: 978-0521575508.
[9] Schwartz, James., In Pursuit of the Gene. From Darwin to DNA. s.l. : Harvard University Press, 2008. ISBN-13: 978-0674026704.
[10] New World Encyclopedia., "Schrödinger, Erwin." New World Encyclopedia. April 24, 2008. http://www.newworldencyclopedia.org/entry/Erwin_Schr%C3%B6dinger.
[ii] Schrödinger’s cat is a thought experiment (see Chapter 37), usually described as a paradox, that Austrian physicist Erwin Schrödinger devised in 1935. It illustrates what he saw as the problem of the Copenhagen interpretation of quantum mechanics applied to everyday objects. The thought experiment presents a cat that might be alive or dead, depending on an earlier random event. In the course of developing this experiment, he coined the term Verschränkung (entanglement).
[iii] Franz Serafin Exner (24 March 1849 - October 15, 1926) was an Austrian physicist.
[iv] Friedrich Hasenöhrl (November 30, 1874 - October 7, 1915) was an Austro-Hungarian physicist.
[v] Arthur Schopenhauer (22 February 1788 – 21 September 1860) was a German philosopher known for his pessimism and philosophical clarity.
[vi] In his lecture "Mind and Matter," Chapter 4, he said that a phrase "that has become familiar to us" is "The world extended in space and time is but our representation (Vorstellung)." This is a repetition of the first words of Schopenhauer’s main work.
[vii] Vedānta was originally a word used in Hindu philosophy as a synonym for that part of the Veda texts known also as the Upanishads.
[viii] Habilitation is the highest academic qualification a scholar can achieve by his or her own pursuit in several European and Asian countries. Earned after obtaining a research doctorate, such as a Ph.D., habilitation requires the candidate to write a professorial thesis (often known as a Habilitationsschrift, or Habilitation thesis) based on independent scholarship, reviewed by and defended before an academic committee in a process similar to that for the doctoral dissertation. However, the level of scholarship has to be considerably higher than that required for a research doctoral (Ph.D.) thesis in terms of quality and quantity, and must be accomplished independently, in contrast with a Ph.D. dissertation typically directed or guided by a faculty supervisor.
[ix] The eigenvectors of a square matrix are the non-zero vectors that, after being multiplied by the matrix, remain proportional to the original vector (i.e., change only in magnitude, not in direction). For each eigenvector, the corresponding eigenvalue is the factor by which the eigenvector changes when multiplied by the matrix. The prefix eigen- is adopted from the German word "eigen" for "own" in the sense of a characteristic description. The eigenvectors are sometimes also called characteristic vectors. Similarly, the eigenvalues are also known as characteristic values.
[x] The Schrödinger equation was formulated in 1926 by Austrian physicist Erwin Schrödinger. Used in physics, specifically quantum mechanics, it is an equation that describes how the quantum state of a physical system changes in time. In the standard interpretation of quantum mechanics, the quantum state, also called a wavefunction or state vector, is the most complete description that can be given to a physical system. Solutions to Schrödinger’s equation describe not only molecular, atomic and subatomic systems, but also macroscopic systems, possibly even the whole universe.
[xi] The quantum harmonic oscillator is the quantum-mechanical analog of the classical harmonic oscillator. Because an arbitrary potential can be approximated as a harmonic potential at the vicinity of a stable equilibrium point, it is one of the most important model systems in quantum mechanics. Furthermore, it is one of the few quantum-mechanical systems for which a simple, exact solution is known.
[xii] The rigid rotor is a mechanical model that is used to explain rotating systems. An arbitrary rigid rotor is a 3-dimensional rigid object, such as a top. To orient such an object in space three angles are required. A special rigid rotor is the linear rotor that is a 2-dimensional object, requiring two angles to describe its orientation. An example of a linear rotor is a diatomic molecule. More general molecules like water (asymmetric rotor), ammonia (symmetric rotor), or methane (spherical rotor) are 3-dimensional, see classification of molecules.
[xiii] Diatomic molecules are molecules composed only of two atoms, of either the same or the different chemical elements. The prefix di- means two in Greek. Common diatomic molecules are hydrogen (H2), nitrogen (N2), oxygen (O2), and carbon monoxide (CO). Seven elements exist in the diatomic state in the liquid and solid forms: H2 , N2, O2, F2, Cl2, Br2, and I2. Most elements (and many chemical compounds) aside from these form diatomic molecules when evaporated, although at very high temperatures, all materials disintegrate into atoms. The noble gases do not form diatomic molecules.
[xiv] The Stark effect (named after Johannes Starck)is the shifting and splitting of spectral lines of atoms and molecules due to the presence of an external static electric field. The amount of splitting and or shifting is called the Stark splitting or Stark shift.
[xv] The Anschluss (spelled Anschluß at the time of the event, and until the German orthography reform of 1996; German for "link-up"), also known as the Anschluss Österreichs, was the occupation and annexation of Austria into Nazi Germany in 1938.
[xvi] Éamon de Valera(14 October 1882 – 29 August 1975) was one of the dominant political figures in twentieth century Ireland, serving as head of government and head of state and introducing the Constitution of Ireland. De Valera was a leader of Ireland’s struggle for independence from Britain in the Irish War of Independence and of the anti-Treaty forces in the ensuing Irish Civil War (1922–23). In 1926, he founded Fianna Fáil and was head of government from 1932–48, 1951–54 and 1957–59 and President of Ireland from 1959–73.
[xvii] Since the 19th century, some physicists have attempted to develop a single theoretical framework that can account for the fundamental forces of nature—a unified field theory. Classical unified field theories are attempts to create a unified field theory based on classical physics. In particular, unification of gravitation and electromagnetism was actively pursued by several physicists and mathematicians in the years between World War I and World War II. This work spurred the purely mathematical development of differential geometry. Albert Einstein is the best known of the many physicists who attempted to develop a classical unified field theory.
[xviii] The negentropy, also negative entropy or syntropy, of a living system is the entropy that it exports to keep its own entropy low; it lies at the intersection of entropy and life. The concept and phrase "negative entropy" were introduced by Erwin Schrödinger in his 1943 popular-science book What is Life?
[xix] Colorimetry is "the science and technology used to quantify and describe physically the human color perception."
Monday, August 1, 2011
Weird Scientist Number 2: Satyendra Nath Bose

Satyendra Nath Bose in Paris 1925
Although more than one Nobel Prize was awarded for research related to the concepts of the boson, Bose–Einstein statistics and Bose–Einstein condensate—the latest being the 2001 Nobel Prize in Physics, which was given for advancing the theory of Bose–Einstein condensates—Bose himself was not awarded the Nobel Prize. Among his other talents, Bose spoke several languages and could also play the esraj, a musical instrument similar to a violin.
In his book, The Scientific Edge, the noted physicist Jayant Narlikar observed (Narlikar, 2003):
“S. N. Bose’s work on particle statistics (c. 1922), which clarified the behaviour of photons (the particles of light in an enclosure) and opened the door to new ideas on statistics of Microsystems that obey the rules of quantum theory, was one of the top ten achievements of 20th century Indian science and could be considered in the Nobel Prize class.”
Early life and career
Bose was born in Calcutta, British India, the eldest of seven children. His father, Surendranath Bose, worked in the Engineering Department of the East Indian Railway Company. Bose attended Hindu School in Calcutta, and later attended Presidency College, also in Calcutta, earning the highest marks at each institution. He came in contact with teachers such as Jagadish Chandra Bose and Prafulla Chandra Roy who provided inspiration to aim high in life. From 1916 to 1921 he was a lecturer in the physics department of the University of Calcutta. In 1921, he joined the department of Physics of the then recently founded Dhaka University (now in Bangladesh and called University of Dhaka) (BANC, 2011).
In 1924, while working as a Reader at the Physics Department of the University of Dhaka, Bose wrote a paper deriving Planck's quantum radiation law without any reference to classical physics and using a novel way of counting states with identical particles. This paper was seminal in creating the very important field of quantum statistics. After initial setbacks to his efforts to publish, he sent the article directly to Albert Einstein in Germany. Einstein, recognizing the importance of the paper, translated it into German himself and submitted it on Bose's behalf to the prestigious Zeitschrift für Physik. As a result of this recognition, Bose was able to leave India for the first time and spent two years in Europe, during which he worked with Louis de Broglie, Marie Curie, and Einstein (Grigrious, 2010).
After his stay in Europe, Bose returned to Dhaka in 1926. He became a professor and was made head of the Department of Physics, and continued teaching at Dhaka University until 1945. He was also Dean of the Faculty of Science at Dhaka University for a long period. When the partition of India became imminent, he returned to Calcutta and taught at Calcutta University until 1956, when he retired and was made professor emeritus (Chatterjee & Chatterjee, 2002).
Bose–Einstein statistics
Bose was an ardent follower of Einstein’s ideas and decided to attempt another derivation of Planck’s law of radiation without using any Maxwellian wave theory of radiation. He had read Planck’s work on the distribution of energy from a black body based on this new theory. Satyendra Nath had always been a perfectionist and would not accept any ad hoc assumption while working out a theory. So he was not happy with Planck’s derivation which had such ad hoc assumptions (Chatterjee & Chatterjee, 2002).
While presenting a lecture at the University of Dhaka on the theory of radiation and the ultraviolet catastrophe, Bose attempted a statistical explanation of the interaction of atoms and radiation using the principle, recently developed by Werner Heisenberg, of uncertainty concerning the characteristics of electrons around the atomic nucleus (Walker A. R., 2011). During this lecture, Bose committed an error in applying the theory, which unexpectedly gave a prediction that agreed with the experiment. He adapted this lecture into a short paper called “Planck's Law and the Hypothesis of Light Quanta”.
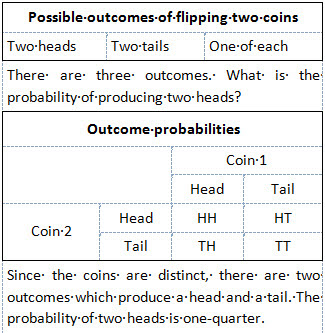
The error was a simple mistake—similar to arguing that flipping two fair coins will produce two heads one-third of the time—that would appear obviously wrong to anyone with a basic understanding of statistics. However, the results it predicted agreed with experiment, and Bose realized it might not be a mistake at all. He for the first time took the position that the Maxwell–Boltzmann distribution would not be true for microscopic particles where fluctuations due to Heisenberg's uncertainty principle will be significant. Thus he stressed the probability of finding particles in the phase space, each state having volume , and discarding the distinct position and momentum of the particles (Grigrious, 2010).
When Bose sent his paper to Philosophical Magazine (Chatterjee & Chatterjee, 2002) for publication it was rejected because the editors thought he had made a simple mistake in his calculations. So he sent a copy to Einstein, soliciting his opinion. (Walker A. R., 2011). Here is what Bose wrote in his first letter to Einstein (Chatterjee & Chatterjee, 2002):
“I have ventured to send you the accompanying article for your perusal and opinion. I am anxious to know what you think of it. You will see that I have tried to deduce the coefficient in Planck’s laws, independent of the classical electrodynamics, only assuming that the ultimate elementary regions in the phase space had the content . I do not know sufficient German to translate the paper. If you think the paper worth publication, I shall be grateful if you arrange for its publication in Zeitschrift fur Physik. Though a complete stranger to you, I do not feel any hesitation in making such a request. Because we are all your pupils though profiting only by your teachings through your writings. I do not know whether you still remember that somebody from Calcutta asked your permission to translate your papers on relativity in English. You acceded to the request. The book has since been published. I was the one who translated your paper ‘Generalised Relativity’.”
Einstein approved. His theory finally achieved respect when Einstein sent his own paper in support of Bose's to Zeitschrift für Physik, asking that they be published together (Walker A. R., 2011). This was done in 1924. Very soon the paper was published, translated by Einstein, and with the following translator’s remark (Chatterjee & Chatterjee, 2002):
“In my opinion Bose’s derivation of the Planck formula signifies an important development. The method considered here yields also the quantum theory of ideal gases which I shall discuss elsewhere.”
The reason Bose's "mistake" produced accurate results was that since photons are indistinguishable from each other, one cannot treat any two photons having equal energy as being two distinct identifiable photons. By analogy, if in an alternate universe coins were to behave like photons and other bosons, the probability of producing two heads would indeed be one-third ( ). But what Bose actually did was more than derive a formula. He introduced new concepts in physics Bose's "error" (Grigrious, 2010), later named as Bose Statistics. Einstein understood the significance of it and immediately applied it to the case of ideal gas and found a new relation known as Bose-Einstein Statistics (Chatterjee & Chatterjee, 2002).
Einstein adopted the idea and extended it to atoms. This led to the prediction of the existence of phenomena which became known as Bose-Einstein condensate, a dense collection of bosons (which are particles with integer spin, named after Bose), which was demonstrated to exist by experiment in 1995 (Grigrious, 2010).
Later work
Bose's ideas were afterwards well received in the world of physics, and he was granted leave from the University of Dhaka to travel to Europe in 1924 (Grigrious, 2010). He spent a year in France and worked with Marie Curie, and met several other well-known scientists. He then spent another year abroad, working with Einstein in Berlin. Upon his return to Dhaka, he was made a professor in 1926. He did not have a doctorate, and so ordinarily he would not be qualified for the post, but Einstein recommended him. His work ranged from X-ray crystallography to unified field theories. He also published an equation of state for real gases with Megh Nad Saha (Chatterjee & Chatterjee, 2002).
When Satyendra Nath returned to Dacca from Europe in 1926, the post of the professor of physics at the Dacca University was vacant. The selection committee recommended the name of D.M. Bose as its first choice and that of S.N. Bose as its alternative choice. D.M. Bose was then the Ghose professor of physics at Calcutta. He was comfortably settled in his research work and was also in close touch with his uncle, Sir J.C Bose and his research laboratory, the Bose Institute, adjacent to the University College of Science and Technology. Since D.M. Bose did not accept the position offered to him by the Dacca University, Satyendra Nath became the professor of physics at Dacca University, where he continued till 1945 (Chatterjee & Chatterjee, 2002).
Apart from physics he did some research in biochemistry and literature (Bengali, English). He made deep studies in chemistry, geology, zoology, anthropology, engineering and other sciences. Being an Indian of Bengali descent, he devoted a lot of time to promoting Bengali as a teaching language, translating scientific papers into it, and promoting the development of the region (Grigrious, 2010).
In 1944 Bose was elected General President of the Indian Science Congress. In 1958 he became a Fellow of the Royal Society.
Works Cited
BANC. (2011). Satyendra Nath Bose . Retrieved August 1, 2011, from Bengali Association of North Carolina: www.banc-online.org/pdfs/SNB.pdf
bosonparticle.com. (2011). Articles about boson particle. Retrieved August 1, 2011, from Boson Particle: http://www.bosonparticle.com/articles
Chatterjee, S., & Chatterjee, E. (2002). Satyendra Nath Bose. National Book Trust.
Grigrious, V. I. (2010, February). Satyendra Nath Bose. Bhouthika Jyothi, 6(Ruby Jubilee Special), p. 41.
Narlikar, J. V. (2003). The Scientific Edge. Penguin Books.
NIC. (2005). Padma Vibhushan Awardees. (National Informatics Centre, Government of India.) Retrieved August 1, 2011, from india.gov.in: http://india.gov.in/myindia/padmavibhushan_awards_list1.php?start=250
Walker, A. R. (2011). From black bodies to bar codes: lasers. In Magical inventions or the art of discovery. Unpublished.
Monday, July 25, 2011
Weird Scientis Number 1: Clinton Davisson
Clinton Davisson
 Davisson, Germer and Calbick in 1927, the year they demonstrated electron diffraction. In their New York City laboratory are Clinton Davisson, age 46; Lester Germer, age 31, and their assistant Chester Calbick, age 23. Germer, seated at the observer's desk, appears ready to read and record electron current from the galvanometer[iii] (seen beside his head); the banks of dry cells behind Davisson supplied the current for the experiments.
Davisson, Germer and Calbick in 1927, the year they demonstrated electron diffraction. In their New York City laboratory are Clinton Davisson, age 46; Lester Germer, age 31, and their assistant Chester Calbick, age 23. Germer, seated at the observer's desk, appears ready to read and record electron current from the galvanometer[iii] (seen beside his head); the banks of dry cells behind Davisson supplied the current for the experiments.
The National Academy of Sciences awarded Davisson the Comstock Prize in 1928. In 1931 Franklin Institute awarded him the Elliott Cresson Medal, and in 1935 the Royal Society (London) awared him the Hughes Medal. In 1941, the University of Chicago awarded him the Alumni Medal. He held honorary doctorates from Purdue University, Princeton University, the University of Lyon and Colby College.
[ii] Sir Owen Willans Richardson, FRS (26 April 1879 - 15 February 1959) was a British physicist who won the Nobel Prize in Physics in 1928 for his work on thermionic emission, which led to Richardson's Law—the current from a heated wire seemed to depend exponentially on the temperature of the wire.
[iii] A galvanometer is a type of ammeter: an instrument for detecting and measuring electric current. It is an analog electromechanical transducer that produces a rotary deflection of some type of pointer in response to electric current flowing through its coil. The term has expanded to include uses of the same mechanism in recording, positioning, and servomechanism equipment.
[iv] Electron optics deals with the focusing and deflection of electrons using magnetic and/or electrostatic fields.
[v] Chystal physics (physical crystallography), is the study of the physical properties of crystals and crystalline aggregates and changes in the properties under the influence of various factors.
Friday, July 15, 2011
General Relativity (excerpt from Albert Einstein: "Nobody expected me to lay golden eggs"
General relativity was Einstein’s theory of gravity, published in 1915 [2], which extended special relativity to take into account non-inertial frames of reference—areas that are accelerating with respect to each other. General relativity takes the form of field equations, describing the curvature of space-time and the distribution of matter throughout space-time. The effects of matter and space-time on each other are what we perceive as gravity.
The theory of the space-time continuum already existed, but under general relativity, Einstein was able to describe gravity as the bending of space-time geometry. Einstein defined a set of field equations, which represented the way that gravity behaved in response to matter in space-time. Physicists could use these field equations to represent the geometry of space-time that was at the heart of the theory of general relativity.
As Einstein developed his general theory of relativity, he had to refine the accepted notion of the space-time continuum into a more precise mathematical framework. He also introduced another principle, the principle of covariance. This principle states that the laws of physics must take the same form in all coordinate systems. In other words, all space-time coordinates are treated the same by the laws of physics—in the form of Einstein’s field equations. This is similar to the relativity principle, which states that the laws of physics are the same for all observers moving at constant speeds. In fact, after general relativity was developed, it was clear that the principles of special relativity were a special case.
Einstein’s basic principle was that no matter where you are—Toledo, Mount Everest, Jupiter, or the Andromeda galaxy—the same laws apply. This time, though, the laws were the field equations, and your motion could very definitely impact what solutions came out of the field equations. Applying the principle of covariance meant that the space-time coordinates in a gravitational field had to work exactly the same way as the space-time coordinates on a spaceship that was accelerating. If you are accelerating through empty space—where the space-time field is flat, as in the left picture of this figure—the geometry of space-time would appear to curve. This meant that if there is an object with mass generating a gravitational field, it had to curve the space-time field as well (as shown in the right picture of the figure).

Without matter, space-time is flat (left), but it curves when matter is present (right).
In other words, Einstein had succeeded in explaining the Newtonian mystery of where gravity came from! Gravity resulted from massive objects bending space-time geometry itself.
Because space-time curved, the objects moving through space would follow the “straightest” path along the curve, which explains the motion of the planets. They follow a curved path around the sun because the sun bends space-time around it.
Again, you can think of this by analogy. If you are flying by plane on Earth, you follow a path that curves around the Earth. In fact, if you take a flat map and draw a straight line between the start and end points of a trip, that would not be the shortest path to follow. The shortest path is actually the one formed by a “great circle” that you’d get if you cut the Earth directly in half, with both points along the outside of the cut. Traveling from New York City to northern Australia involves flying up along southern Canada and Alaska—nowhere close to a straight line on the flat maps we are used to.
Similarly, the planets in the solar system follow the shortest paths—those that require the least amount of energy—and that results in the motion we observe.
In 1911, Einstein had done enough work on general relativity to predict how much the light should curve in this situation, which should be visible to astronomers during an eclipse [3].
When he published his complete theory of general relativity in 1915, Einstein had corrected a couple of errors [249]. In 1919, an expedition set out to observe the deflection of light by the sun during an eclipse, in to the west African island of Principe. The expedition leader was British astronomer Arthur Eddington, a strong supporter of Einstein [3].
Eddington returned to England with the pictures he needed, and his calculations showed that the deflection of light precisely matched Einstein’s predictions. General relativity had made a prediction that matched observation.
Albert Einstein had successfully created a theory that explained the gravitational forces of the universe and had done so by applying a handful of basic principles. To the degree possible, the work had been confirmed, and most of the physics world agreed with it. Almost overnight, Einstein’s name became world famous. In 1921, Einstein traveled through the United States to a media circus that probably was not matched until the Beatlemania of the 1960s [4].
References
[1] Einstein, Albert., The Theory Of Relativity . s.l. : Citadel, 2000 [1949]. ISBN-13: 978-0806517650.
[2] Einstein, Albert., "Die Feldgleichungen der Gravitation." Sitzungsberichte der Preussischen Akademie der Wissenschaften zu Berlin. November 25, 1915, pp. 844–847. http://nausikaa2.mpiwg-berlin.mpg.de/cgi-bin/toc/toc.x. cgi?dir=6E3MAXK4&step=thumb.
[3] Einstein, Albert., "Über den Einfluß der Schwerkraft auf die Ausbreitung des Lichtes (About the influence of gravity on the propagation of light )." Annalen der Physik. 1911, Vol. 340, 10. translated "On the Influence of Gravitation on the Propagation of Light" in The collected papers of Albert Einstein. Vol. 3 : The Swiss years: writings, 1909–1911 (Princeton University Press, Princeton, NJ, 1994), Anna Beck translator.
[4] Pais, Abraham., Subtle is the Lord. The Science and the Life of Albert Einstein. s.l. : Oxford University Press, 1982. pp. 382–386. ISBN 019853907X
Thursday, July 14, 2011
Using Math to Defeat the Enemy
This book is organized in two parts: Simulation (Part I) and Modeling (Part II). There are numerous practical applications and example models used in past and current military simulations.
Jeffrey S. Strickland, Ph.D.
CMSP, ASEP
President
Simulation Educators
Colorado Springs, Co
www.simulation-educators.com
Tuesday, July 12, 2011
Chaos Theory for Beginners (excerpt from Quantum Phaith)
However, there is another reason that Chaos Theory was born so recently, and that is the ‘Quantum Mechanical Revolution’ and how it ended the ‘deterministic’ era.
[1] "It's a Jungle out There" is a song written by Randy Newman, the theme song for the TV series Monk since its second season. In 2004, it won an Emmy Award for best theme song.
Sunday, July 10, 2011
Exerpt from new book: "Quantum Phaith"
There is a verse in Scripture that many know or have heard (even people who have never picked up a Bible). The New American Standard translation of Luke 17:6 says: